Abstract
Background: Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia, characterized by chronic hemolysis mediated by classical complement pathway activation. The ongoing COVID-19 pandemic has posed an additional challenge in treating patients with CAD. Anti-CD20 therapies (commonly used off-label to treat CAD) increase the risk of serious COVID-19 infection and reduce the immune response to COVID-19 vaccines. Recent evidence has also shown a correlation between CAD exacerbations and both COVID-19 and COVID-19 vaccines; raising concern amongst clinicians as CAD patients are usually elderly with comorbidities and will likely require additional boosters during the ongoing pandemic. Sutimlimab (SUT), recently FDA approved for patients with CAD, is a first-in-class humanized monoclonal antibody which selectively targets C1s in the classical complement pathway; addressing the underlying mechanism of hemolysis in CAD.
In the Phase 3 CARDINAL (NCT03347396) and CADENZA (NCT03347422) studies, SUT treatment led to rapid and sustained increases in hemoglobin and clinically meaningful improvements in fatigue and was generally well tolerated - reported adverse events (AE) were consistent with elderly populations. Both studies spanned the period before and during the pandemic; investigators were advised to vaccinate enrolled patients without stopping SUT treatment. However, the impact of SUT on immune response to COVID-19 vaccination is unknown.
Objective: To explore concomitant use of COVID-19 vaccines and SUT in patients with CAD
Methods: Post-hoc analysis from the open label extension (OLE) part of the CADENZA and CARDINAL studies. COVID-19 cases/diagnoses were collected via the regular AE reporting system. Data on tolerability to COVID-19 vaccine was collected through AE reports. Immunogenicity to COVID-19 vaccines was analyzed in a subset of patients fully vaccinated (2 doses) against COVID-19 and had consented to the use of stored samples (collected no later than 6 months after vaccination). Anti-Sars-CoV-2 Spike (EUROIMMUN Anti-SARS-CoV-2 ELISA) and anti-Sars-CoV-2 Nucleocapsid (Abbott Architect i2000 SR) IgG titers were measured. Three patients who tested positive for COVID-19 during Part A of both studies were excluded from the immunogenicity analysis.
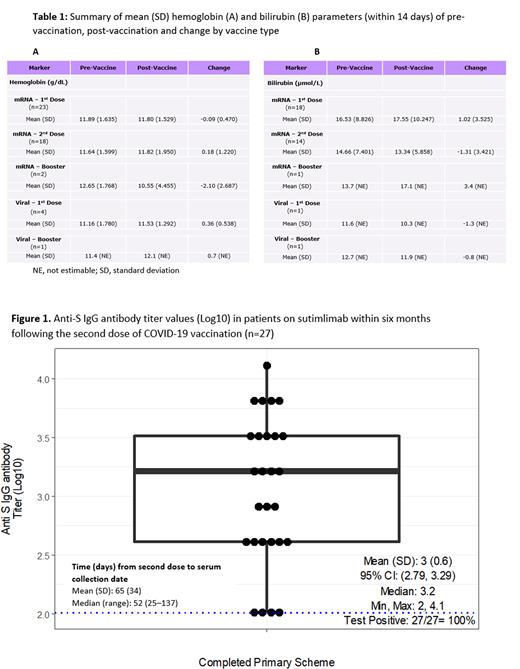
Results: Of the 61 completers from both studies, 47 received at least one dose of a COVID-19 vaccine; 14 received none; 11 had an additional booster. All participants, regardless of vaccination status, had similar demographic and clinical characteristics. Among those vaccinated, most (83%) received both doses during the studies; of these, mRNA vaccines were the most common (79%). The mean inter-dose interval for fully vaccinated patients (n=37) was 38 days; those who received 1 booster (n=11) had a mean of 165 days between the last dose and booster. No COVID-19 cases were reported during the OLE. Ten AEs were reported in 8 patients during the 7 days after any vaccine dose, but none more than once, and there were no serious AEs. SUT therapy was postponed/skipped twice due to vaccine administration, at the investigator's discretion. Anemia and hemolysis markers, including hemoglobin and total bilirubin, were measured after vaccination to assess its impact on hemolytic activity. No indications of hemolytic exacerbation were observed (Table 1). The immunogenicity analysis comprised 27 patients. All patients developed an immune response post-vaccination, measured via positive IgG anti-spike antibodies (Figure 1). The mean time between last dose and serum collection was 65 days (range: 25-137). Antibody titers were similar irrespective of gender or age. A further analysis performed on 6 patients with booster vaccinations demonstrated elevated immune responses pre to post booster (mean [95% CI] log10 titer change: 1.82 [0.79-2.85]). To rule out previous asymptomatic COVID-19 infection, anti-nucleocapsid SARS-Cov-2 IgG antibodies were assayed and were negative in all cases.
Conclusions: COVID-19 vaccination response was not impaired in patients with CAD being treated with SUT. COVID-19 vaccines were well tolerated, and there were no signs of increased hemolytic marker activity following COVID-19 vaccination in patients receiving SUT.
Disclosures
Fattizzo:Alexion: Consultancy, Speakers Bureau; Amgen: Consultancy; Sobi: Speakers Bureau; Janssen: Consultancy; Momenta: Consultancy; Novartis: Consultancy, Speakers Bureau. Roeth:Roche: Consultancy, Honoraria, Research Funding; Biocryst: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Apellis Pharmaceuticals: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding. Broome:Sanofi: Honoraria, Other: Participation on a Data Safety Monitoring Board or Advisory Board , Research Funding; Alexion: Other: Participation on a Data Safety Monitoring Board or Advisory Board, Speakers Bureau; Argenx: Other: Participation on a Data Safety Monitoring Board or Advisory Board, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Rigel: Research Funding. Khan:Sanofi: Current Employment, Current equity holder in private company; Seattle Children's Hospital: Ended employment in the past 24 months. Shafer:Sanofi: Current Employment. Cordoba:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Barcellini:Apellis: Honoraria; Biocryst: Honoraria; Alexion: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Momenta: Honoraria; Novartis: Honoraria; SOBI: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Research Funding; Sanofi: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.